Risk assessment of genetically modified plants

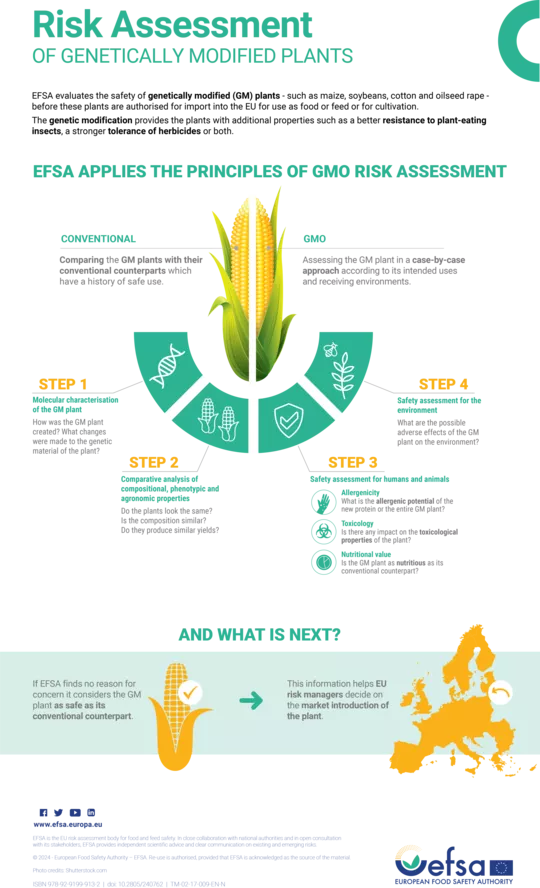
EFSA evaluates the safety of genetically modified (GM) plants – such as maize, soybeans, cotton and oilseed rape – before these plants are authorised for import into the EU for use as food or feed or for cultivation.
The genetic modification provides the plants with additional properties such as a better resistance to plant-eating insects, a stronger tolerance of herbicides or both.
EFSA applies the principles of GMO risk assessment
Conventional
Comparing the GM plants with their conventional counterparts which have a history of safe use.
GMO
Assessing the GM plant in a case-by-case approach according to its intended uses and receiving environments.
Step 1
Molecular characterisation of the GM plant
How was the GM plant created? What changes were made to the genetic material of the plant?
Step 2
Comparative analysis of compositional, phenotypic and agronomic properties
Do the plants look the same? Is the composition similar? Do they produce similar yields?
Step 3
Safety assessment for humans and animals
- Allergenicity - What is the allergenic potential of the new protein or the entire GM plant?
- Nutritional value - Is the GM plant as nutritious as its conventional counterpart?
- Toxicology - Is there any impact on the toxicological properties of the plant?
Step 4
Safety assessment for the environment
What are the possible adverse effects of the GM plant on the environment?
And what's next?
EFSA shares its conclusions on the safety of the GM plant with EU risk managers.
This information helps EU risk managersdecide on the market introduction of the plant.