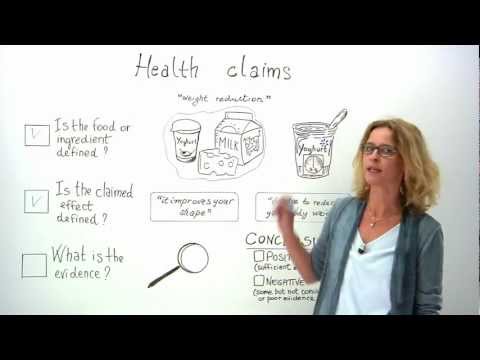
Health claims

An increasing number of foods sold in the EU bear nutrition The science of how diet relates to the body's need for sustenance and health claims. A nutrition claim A statement that implies that a foodstuff has beneficial nutritional properties, such as being “low fat” or “high in fibre” states or suggests that a food has beneficial nutritional properties, such as “low fat”, “no added sugar” and “high in fibre”. A health claim Any practice (e.g. a statement or visual) used in food marketing to suggest that health benefits can be gained from consuming a given food, nutrient or ingredient is any statement on labels, advertising or other marketing products that health benefits can result from consuming a given food, for instance that a food can help reinforce the body’s natural defences or enhance learning ability.

Ongoing and completed assessments
Follow EFSA's work
EFSA's role
EFSA’s work includes providing scientific advice on:
- General function health claims under Article 13.1 of the EU Regulation
- Claims regarding disease risk reduction and child development or health under Article 14 of the EU Regulation
- Criteria for setting nutrient profiles
EFSA is responsible for verifying the scientific substantiation of the submitted claims, some of which are currently in use, some of which are proposed by applicants – companies who want to submit claims for authorisation in the EU. This information serves as a basis for the European Commission and Member States, which will then decide whether to authorise the claims.
Guidance for applicants and engagement with stakeholders
EFSA has prepared guidance on how to submit claims applications, following an extensive consultation process with industry and other interested parties.
EFSA has been engaging regularly with stakeholders to outline and clarify where needed the process followed by the NDA Panel in the evaluation of claims, and has been providing guidance in this field since 2007 through guidance, briefing documents and the holding of scientific meetings.
EU framework
In December 2006 EU decision makers adopted a Regulation on the use of nutrition and health claims for foods which lays down harmonised EU-wide rules for the use of health or nutritional claims on foodstuffs based on nutrient An element or compound needed for normal growth, development and health maintenance. Essential nutrients cannot be made by the body and must, therefore, be consumed from food profiles. Nutrient profiles are nutritional requirements that foods must meet in order to bear nutrition and health claims. One of the key objectives of this Regulation is to ensure that any claim made on a food label in the EU is clear and substantiated by scientific evidence.
- Regulation 1924/2006 on nutrition and health claims made on foods
- Commission Regulation No 353/2008 establishing implementing rules for applications for authorisation of health claims
- Commission Regulation No 1169/2009 amending Regulation (EC) No 353/2008 establishing implementing rules for applications for authorisation of health claims
FAQ
A health claim is any statement used on labels, in marketing or in advertising that health benefits can result from consuming a given food or from one of its components such as vitamins and minerals, fibre, and ‘probiotic’ bacteria. There are different types of health claims. For instance, statements that a food can help reinforce the body’s natural defences or enhance learning ability are called “general function” claims. Examples also include claims on the reduction of disease risk and other substances that may improve or modify the normal functions of the body, e.g. “Plant sterol have shown to reduce cholesterol levels, a risk factor in the development of coronary heart disease” or “Calcium may help improve bone density”.
A nutrition claim states or suggests that a food has particular beneficial nutritional properties. Examples include “low fat”, “source of omega-3 fatty acids” or “high in fibre”.
The Regulation on Nutrition and Health Claims on Foods requires that foods bearing nutrition and health claim must meet certain nutritional requirements or so-called “nutrient profiles.” Foods need to comply with these conditions in order to be eligible to make such claims. The profiles will help ensure that consumers who utilise claims to guide healthy diet choices, and who may perceive foods bearing claims as having a nutritional or health advantage, are not misled as to their overall nutritional value. The European Commission and Member States will establish a nutrient profiling system and set nutrient profiles for foods bearing nutrition and health claims taking into account EFSA’s scientific advice.
- To give advice on establishing a positive list of permitted health claims
The European Commission is required to draw up a “positive list” of the many well-established “general function” health claims in the EU, such as “calcium is good for your bones”, on the basis of claims submitted by the EU Member States. This type of health claims, dealt with under Article 13.1 of the Regulation, include those referring for instance to growth, development and the functions of the body and to psychological and behavioural functions, but not to the reduction of disease risk nor to child development or health which are separately addressed under Article 14 and Article 13.5 of the Regulation. EFSA is providing scientific advice to support this process. So far, EFSA has published 125 opinions providing scientific advice for more than 900 health claims, out of the draft a list of 4,637 health claims submitted to EFSA by the Commission between July 2008 and March 2010. The list of authorised health claims will be adopted progressively by the European Commission and Member States taking into account EFSA’s opinions.
More information on Article 13.1 claims - To give an opinion on individual applications for health claims
New “function” health claims
In addition to those claims included in the positive list of permitted “general function” health claims, applicants can submit dossiers to the Member States in order to seek a risk assessment A specialised field of applied science that involves reviewing scientific data and studies in order to evaluate risks associated with certain hazards. It involves four steps: hazard identification, hazard characterisation, exposure assessment and risk characterisation from EFSA on new function claims for a specific product. For these claims which are based on newly developed scientific evidence, protection of proprietary data can be requested. These claims as referred to in Article 13.5 of the Regulation, will be transmitted to EFSA by competent authorities in Member States and will be assessed on a case-by-case basis by EFSA’s NDA Panel. The applicants shall present more complete evidence in a structured manner for their specific products. EFSA is required to deliver these opinions within five months.
Summary of Art 13.5 claims applications received by EFSA
Claims regarding disease risk reduction and child development or health
These include claims relating to reduction of disease risk or to child development or health, which are dealt with under Article 14 of the Regulation. Any such claims submitted for inclusion in the EU positive list have to be examined by EFSA and approved by the Commission and Member States. EFSA is to verify that the health claim is substantiated by scientific evidence, delivering its opinion within 5 months of validating the applications received. The applicant is requested to present more complete evidence in a structured manner for their specific products.
Summary of Art 14 claims applications received by EFSA - To provide guidance on the preparation of applications for the authorisation of health claims
EFSA is an organisation committed to openness, transparency and dialogue. One of the ways it demonstrates this commitment is through its activities with stakeholders. In July 2007, EFSA’s NDA Panel has published guidance for applicants on the submission of health claims for authorisation. The guidance, developed following public consultation, aims to help companies who want to submit health claims for authorisation. It addresses the types of information to include in the application, in particular concerning the scientific data and evidence required to support claims. In September 2009, EFSA has also provided further guidance to applicants in this area by publishing a revised Frequently Asked Questions (FAQs) document. This document takes into account the experience gained with the evaluation of health claims applications, comments received through the consultation held in May 2009, as well as the comments received from applicants at a technical meeting in June 2009 on Article 14 and 13.5 health claims.
How to submit an application - To give scientific advice on nutrient profiles
EFSA’s task is to provide scientific advice that could be used by EU policy makers in establishing nutrient profiles. EFSA’s NDA Panel delivered the main elements of its scientific advice in a Scientific Opinion Opinions include risk assessments on general scientific issues, evaluations of an application for the authorisation of a product, substance or claim, or an evaluation of a risk assessment on nutrient profiles adopted on 31 January 2008. This first step will help inform the Commission and Member States in deciding which nutrient profile system to implement. EFSA will continue supporting the Commission in its ongoing work to establish a final nutrient profiling system. To this end, EFSA developed a new tool to assist EU risk managers in testing different nutrient profile scenarios - a tailor-made food composition database compiled in co-operation with Member States and industry. The database was delivered to the European Commission in July 2008.
EFSA regularly updates its website with information on progress of work in the area of claims. The most up-to-date information is available on EFSA's website.
The European Commission published comprehensive information on the objectives and scope of the Regulation on health and nutrition claims, as well as the many issues surrounding it.
European Commission: Questions and Answers on health and nutrition claims